Other Reports
The intial opening of the Reports page also opens a Report Settings popup window for the default report, the CTRP Data Table 4 Report. In the Report Settings window,the Data Grid for the report can be tailored according to the settings widget in the window. Though the CTRP Data Table 4 Report is the default report, there are other reports that can be run as well.
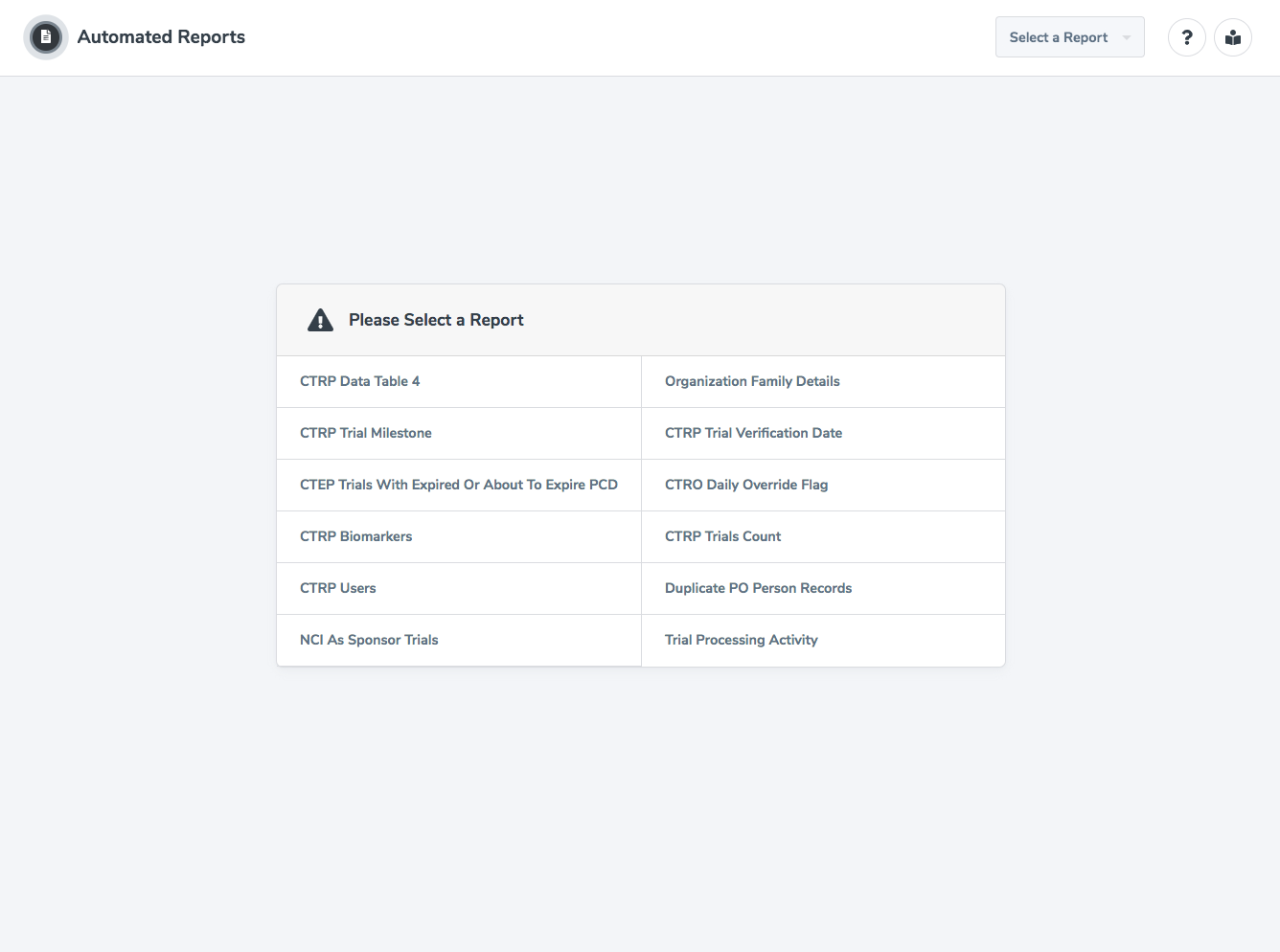
Here is a listing of all of the reports that accompany the CTRP Data Table 4 Report:
- Organization Family Details
- CTRP Trial Milestone
- CTRP Trial Verification Date
- CTRP Trials With Expired Or About To Expire PCD
- CTRO Daily Override Flag
- CTRP Biomarkers
- CTRP Trials Count
- CTRP Users
- Duplicate PO Person Records
- NCI As Sponsor Trials
- Trial Processing Activity
The Reports Settings for the reports on the CTRP Data Table 4 Reports page are standard settings controls. There are different dropdown picklists according to which report type is chosen.
Once the report has been run, a Data Grid is displayed with the corresponding records. There are additional settings that can be configured using the controls on the main page. One of these controls is a drop-down box that allows the user to switch between reports.
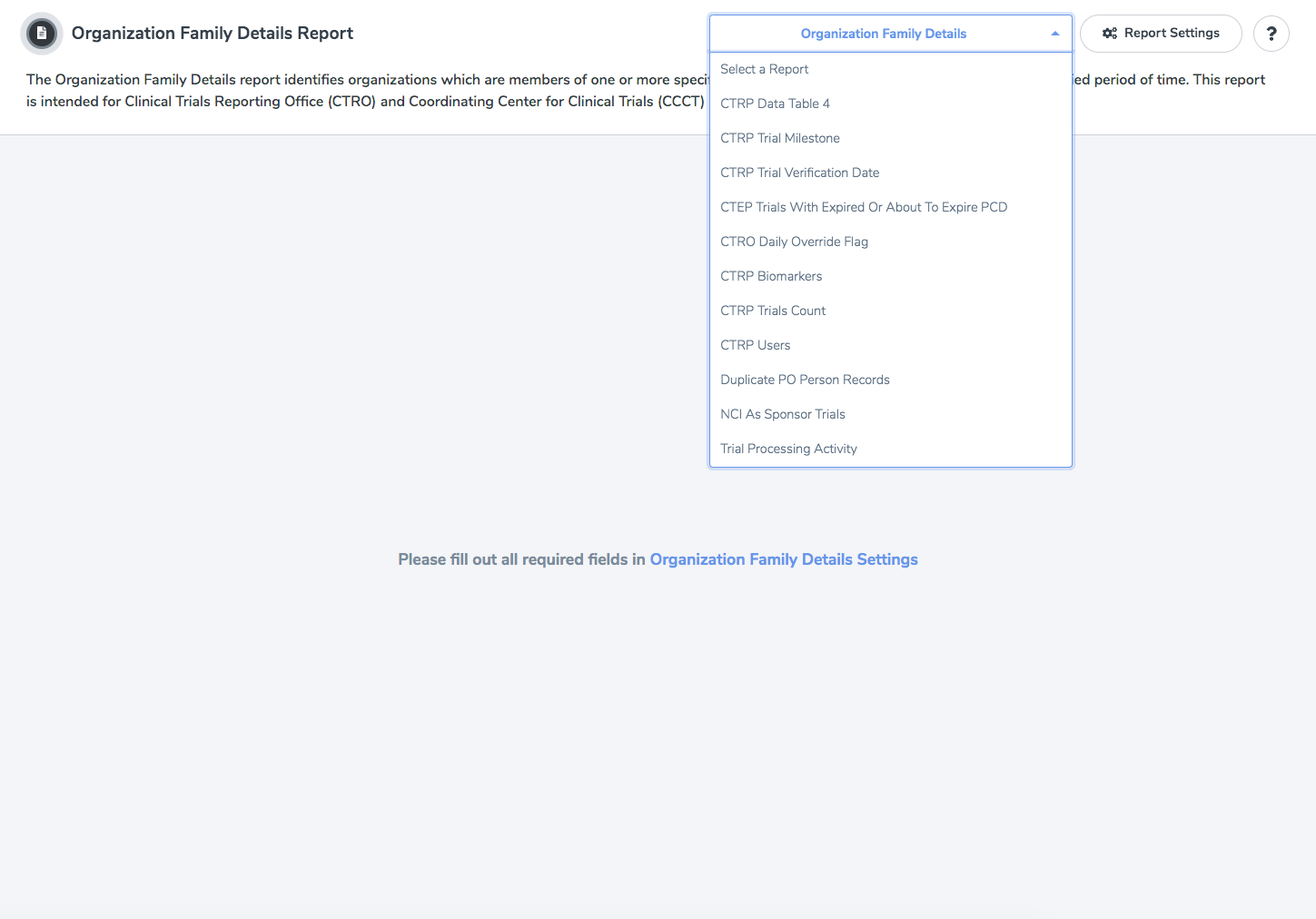
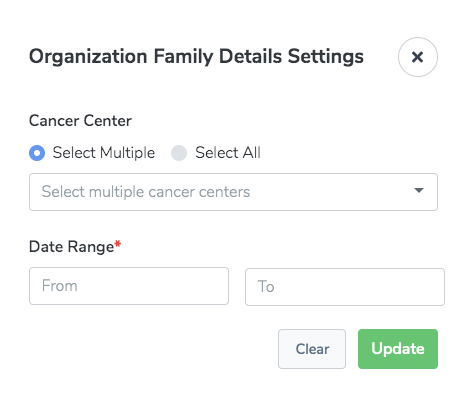
Organization Family Details
The Organization Family Details report identifies organizations which are members of one or more specified Cancer Center organization families during a specified period of time. This report is intended for Clinical Trials Reporting Office (CTRO) and Coordinating Center for Clinical Trials (CCCT) users.
There are settings specific to this report:
CTRP Trial Milestone
The CTRP Trial Milestone report provides details on all the milestones added to trials by CTRO in the CTRP PA application in a specified time period. The milestones show the progress of CTRO in reviewing and abstracting the trial. Typically, CTRO specifies one month when running this report, but the report allows you to specify any time period. This report is intended for Clinical Trials Reporting Office (CTRO) and Coordinating Center for Clinical Trials (CCCT) users.
There are settings specific to this report:
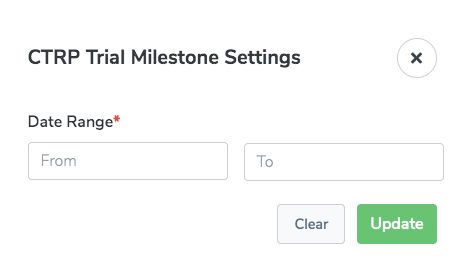
CTRP Trial Verification Date
The CTRP Trial Verification Date report provides details on trials with a verification date older than six months. This report is intended for Clinical Trials Reporting Office (CTRO) and Coordinating Center for Clinical Trials (CCCT) users.
There are no additional settings for this report.
CTEP Trials With Expired Or About To Expire PCD
The CTEP Trials with expired or about to expire PCD report identifies CTEP Trials where the Primary Completion Date (PCD) has expired or is about to expire (within the next 90 calendar days). This report is intended for Clinical Trials Reporting Office (CTRO) and Coordinating Center for Clinical Trials (CCCT) users.
There are no additional settings for this report.
CTRO Daily Override Flag
The CTRO Daily Override Flag report indicates for each trial, whether the system sends the trial information to ClinicalTrials.gov in the automated nightly batch updates (via FTP). This report is intended for Clinical Trials Reporting Office (CTRO) and Coordinating Center for Clinical Trials (CCCT) users.
There are no additional settings for this report.
CTRP Biomarkers
The CTRP Biomarkers report lists all trials registered in the CTRP that meet the biomarker attribute or set of biomarker attributes you select. This report is intended for Clinical Trials Reporting Office (CTRO) and Coordinating Center for Clinical Trials (CCCT) users.
There are settings specific to this report:
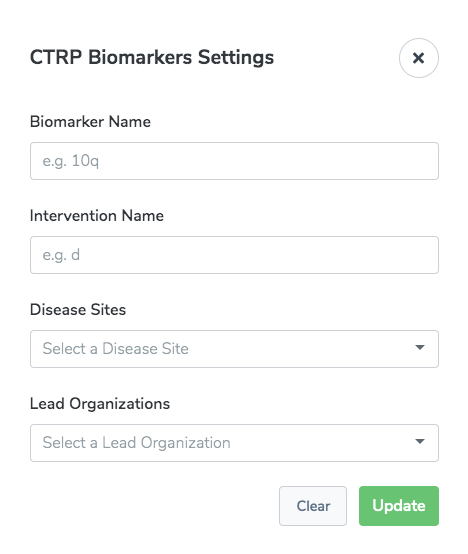
CTRP Trials Count
The CTRP Trials Count report provides the number of trials to date in each of several trial types and statuses. This report is intended for Clinical Trials Reporting Office (CTRO) and Coordinating Center for Clinical Trials (CCCT) users.
There are no additional settings for this report.
CTRP Users
The CTRP Users report provides information about CTRP users. This report is intended for Clinical Trials Reporting Office (CTRO) and Coordinating Center for Clinical Trials (CCCT) users.
There are no additional settings for this report.
Duplicate PO Person Records
The Duplicate PO Person Records report lists pairs of PO person records that have been identified by the system as possible duplicates. This report is intended for Clinical Trials Reporting Office (CTRO) and Coordinating Center for Clinical Trials (CCCT) users.
There are no additional settings for this report.
NCI As Sponsor Trials
The NCI as Sponsor Trials report provides information about the records of NCI-sponsored trials. This report is intended for Clinical Trials Reporting Office (CTRO) and Coordinating Center for Clinical Trials (CCCT) users.
There are no additional settings for this report.
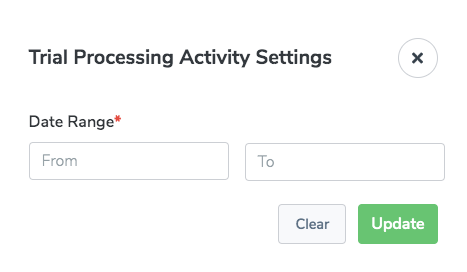
Trial Processing Activity
The Trial Processing Activity report provides the number of trials in each of several trial types and statuses, based on a specified date range. This report is intended for Clinical Trials Reporting Office (CTRO) and Coordinating Center for Clinical Trials (CCCT) users.
There are settings specific to this report: